|
HOME >>
Pharmaceuticals API List 2 >>
Pantoprazole Sodium

Product Name :
PANTOPRAZOLE SODIUM
SALT

C.A.S. No:
138786-67-1
Synonyms :
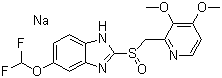
Sodium-[5-(Difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyri - dinyl)-methyl]-sulfinyl]-1H-benzimidazolide
Molecular Formula:
C16H14F2N3NaO4S1.5
H2O
Formula Weight :
432.4
Product Group:
Antiulcerative
Pantoprazole Sodium
is a substituted benzimidazole proton-pump inhibitor (PPI) that
suppresses gastric acid secretion by inhibiting the gastric (H+,K+)-ATPase
enzyme pump. Pantoprazole Sodium forms a covalent bond to two sites
of the (H+,K+)-ATPase enzyme system at the secretory surface of the
gastric parietal cell; this binding results in antisecretory effects
that persists for more than 24 hours. A significant increase in
gastric pH and decrease in basal acid output follow oral and IV
administration of Pantoprazole Sodium. Pantoprazole Sodium does not
antagonize H2 or cholinergic receptors.
Most studies report that gastric acid suppression is dose-related
following IV and oral administration of Pantoprazole Sodium,
resulting in inhibition of both basal and stimulated gastric acid
secretion (irrespective of the stimulus).
Significant in vitro activity against Helicobacter pylori (H.
Pylori) has been demonstrated for Pantoprazole Sodium.
Similar to omeprazole and other PPIs, hypergastrinemia can occur
during Pantoprazole Sodium therapy. Although prolonged
hypergastrinemia has been associated with gastric tumors in rats,
long-term studies of proton pump inhibitors do not suggest the
development of tumors in humans.
No clinically relevant effects of Pantoprazole Sodium on
cardiovascular, respiratory, ophthalmic, or central nervous system
function have been detected. A short-term (2-week) evaluation showed
that oral Pantoprazole Sodium had no effect on endocrine hormones
including: cortisol, testosterone, triiodothyronine (T3), thyroxine
(T4), thyroid-stimulating hormone, thyronine-binding protein,
parathyroid hormone, insulin, glucagon, renin, aldosterone,
follicle-stimulating hormone, luteinizing hormone, prolactin and
growth hormone.
HAZARDS IDENTIFICATION
Not considered hazardous when handled under normal
conditions with good house keeping.
FIRE
FIGHTING MEASURES
Extinguishing
Media:
Carbon
dioxide, dry chemical powder or appropriate foam. Water spray.
Unusual
fire and explosion hazards:
Emits toxic fumes under fire conditions
Special fire fighting procedures:
Wear
self-contained breathing apparatus and protective clothing to
prevent contact with the skin and eyes.
Accidental
Release Measures:
Ensuring
personal safety, mark out contaminated area with signs and prevent
unauthorized access. Turn leaking containers up to prevent further
Evaculate area. Wear self-contained breathing apparatus, rubber
boots & heavy rubber gloves. Sweep-up/absorb in suitable material,
place in a container and hold for disposal. Avoid raising dust.
Ventilate area and wash spill site after pick-up complete.
Handling and Storage:
Store in original container in a cool dark place.
EXPOSURE CONTROL/PERSONAL
PROTECTION:
Respiratory protection:
required when dusts are generated.
Eye protection:
required.
Hand protection:
required.
Industrial hygiene:
Immediately change contaminated clothing. Wash hands and face after
working with substance.
PHYSICAL AND
CHEMICAL PROPERTIES:
Appearance
White to off-white powder
Ecological Information
Do not allow product to enter drinking water supplies, waste water
or soil!
DISPOSAL CONSIDERATIONS:
Dissolve or
mix material with a suitable combustible solvent and incinerate in a
chemical incinerator equipped with an afterburner and scrubber.
Material should be disposed of in keeping with all local and
national legislation. Packaging should be disposed of in keeping
with all local and national legislation. Handle contaminated
containers as product.
TRANSPORT
INFORMATION:
Classification:
UN Number:
While the information herein is believed to be reliable, it is
furnished without warranty of any kind. It shall be used only as a
guide. We assume no liabilities from the use of this product or
information contained herein.
We have
experience in
Exporting and Manufacturing of
all
Countries and Overseas medicines
in
quick reliable manner and we are very interested to start
collaboration with your company or organisation!
2004 - 2019 Taj Pharmaceuticals Limited. All rights reserved
Note:-We are committed to helping you find the right answers to your
questions and concerns. However, this web site is not intended to
give investment advice, promote the use of Taj Pharmaceuticals
Ltd products or provide information on which to base medical
treatment. If you have questions regarding any Taj Pharmaceuticals
Ltd product or are experiencing a medical emergency, please consult
your health care provider.
Active Pharmaceutical Ingredients
manufacturer, exporter, drug ingredients, pharmaceuticals, India
Additionally, contact information on this web site cannot be used to
report adverse drug events. If you are a physician, please follow
the procedures required by your country's regulations. Please choose
one of the given options to contact us and we will respond to

|











